Gene medicine offers hope to those who have a rare genetic disorder
Such efforts will help parents to overcome potential obstacles. They must be experts in drug development, raising millions of children, and securely sound in science. Few people can get rid of it.
“There are a lot of people who know how to do genetic therapy, but the knowledge is shared, and a lot can be confusing,” says Sanath Kumar Ramesh, a programmer whose son suffers from a rare disease. Ramesh set up an organization, Open Help, which develops programs that families can use to develop clinical research, including methods such as recruiting scientists to create species of infected animals.
“I think in the future, the differences between scientists and parents will not be clear,” he says.
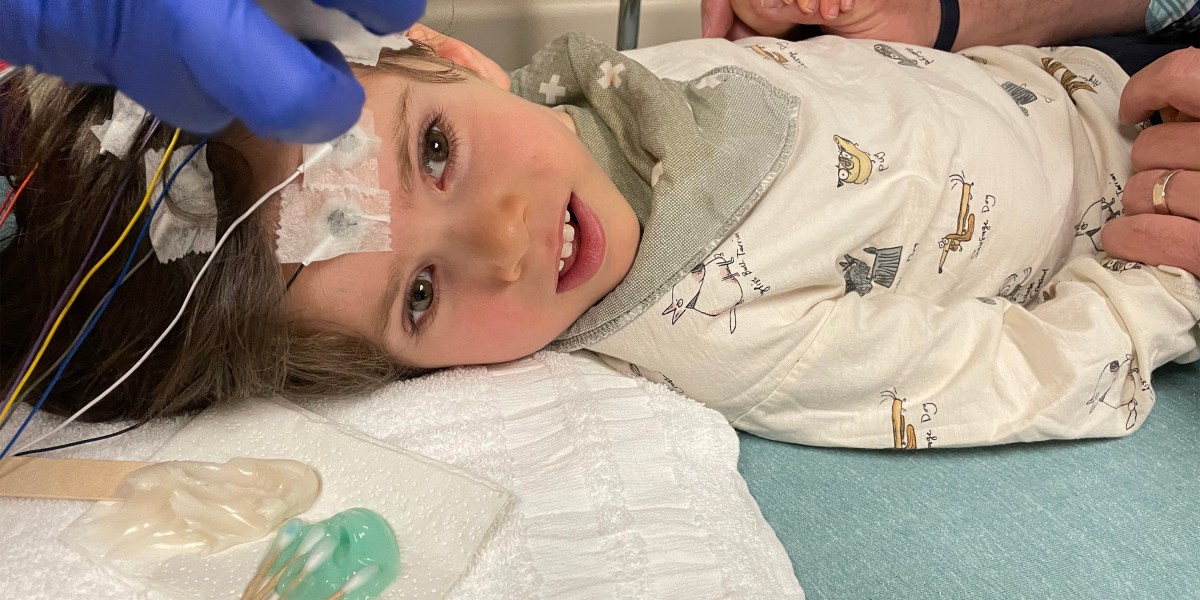
For parents whose children have already been accepted in the Dayton trial, genetic therapy may be their last resort. One of them is Meagan Rockwell, a nail artist from Cedar Rapids, Iowa, whose daughter, Tobin Chisomo, who is now half and half, was acquired by Canavan in 2018.
“She told us sorry, there is nothing we can do – no treatment, no treatment – you will be lucky if she sees a birthday. It was very painful, to know that your only child has a life-threatening brain disorder,” said Rockwell.
Rockwell says he became aware of Leone’s online drug efforts and eventually raised more than $ 250,000. “At the time, Tobin was very young in the US and Kanavan’s, and I think that helped him a lot,” he says, adding that Leone tells parents that money puts them first but does not guarantee support.
Bateman-House, a medical scientist, says another risk factor is that if parents can judge the merits of the experiment in an “impartial” way, especially if they have invested a lot of money in the profession. “Not only is their child vulnerable; and that their blood, sweat, and tears are what help them, ”he said. “It would be very difficult for a parent to change their mind and say, ‘We don’t do this.'”
Hope versus risk
Dayton’s study currently has enough drugs to treat only nine or ten children. It was developed in Spain, but after researchers and families have overcome what they call the problem of red tape, delays, and barriers, some are thrown out by government officials who choose alternative therapies and if the trials are well-designed.
Sometime in 2019, Landsmans took their sons to the U.S. Food and Drug Administration at a conference they attended after inviting several lawmakers. “In the past we had a case number on their large stack of papers,” says Jennie Landsman, the boys’ mother. “They had technical objections. At the meeting we encouraged Benny and Josh, and we said ‘We hope the issue that’s the smartest doesn’t stop the drug.’ ”
THE STORY OF JENNIE LANDSMAN
Dayton’s trial came to a head in December and began shortly after Benny, who will turn five in June. “Benny is the pilot. Benny is’ God, we hope this baby works, ” said Rockwell, who does not have a date for his son.
What are the opportunities? Gene methods have been very successful, healing children who are not immune, and prevention of mental illness. From 2017, small number of genetic therapies approved for sale in the US, at prices totaling $ 2.1 million every child.
Writing prices have attracted the attention of industry-leading professionals, who are now seeing the business even in the most rare cases. One, called Aspa Therapeutics, is said to have the idea to trigger various experiments on Kanavan’s genes. Their chief executive, Eric David, estimates that there are 1,000 infected children in the US and Europe. “This, for us, is enough,” he says.
There is no definite gene therapy that will work well in Kanavan. Although genetically modified genes may prevent the progression of the disease, children’s brains may have been damaged long ago.
Source link